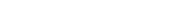About BLMo-308
About BLMo-308
alopecia but also to hair loss induced by inflammation.
alopecia but also to hair loss induced by inflammation.
Features of BLMo-308
A Different Mechanism
than Finasteride.
Rather than directly targeting the hormonal mechanism of the male hormone testosterone, it works by inhibiting the production of the inflammatory substance IL-6.
No Side Effects
It is an ingredient extracted, concentrated, and blended from food-grade plant ingredients.
No Adverse Events Observed during 6 Months of Administration of BLMo-308 in a Clinical Trial
Clinical Trial Completed.
The clinical trial completion report for the ingredient was submitted at the end of 2022
A different mechanism than finasteride.
Rather than directly targeting the hormonal mechanism of the male hormone testosterone, it works by inhibiting the production of the inflammatory substance IL-6.
No side effects
It is an ingredient extracted, concentrated, and blended from food-grade plant ingredients.
Clinical trial completed.
The clinical trial completion report for the ingredient was submitted at the end of 2022
A different mechanism than finasteride.
Rather than directly targeting the hormonal mechanism of the male hormone testosterone, it works by inhibiting the production of the inflammatory substance IL-6.
No side effects
It is an ingredient extracted, concentrated, and blended from food-grade plant ingredients.
No Adverse Events Observed during 6 Months of Administration of BLMo-308 in a Clinical Trial
Clinical trial completed.
The clinical trial completion report for the ingredient was submitted at the end of 2022
BACKGROUND KNOWLEDGE
Androgenetic alopecia and Finasteride
75% of male hair loss patients and 40% of female hair loss patients have androgenetic alopecia.
What is androgenetic alopecia?
The main cause responsible for androgenetic alopecia is dihydrotestosterone (DHT), a more potent form of testosterone. Testosterone, a male hormone, is converted into DHT by a specific enzyme called 5-alpha reductase. DHT binds to hair follicles, causing them to shrink and shorten their growth cycle. This ultimately results in the gradual thinning and eventual loss of hair.
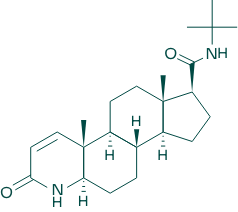
As a solution to this, Merck & Co. developed ‘finasteride,’ with the idea that reducing DHT production by inhibiting the activity of 5-alpha reductase would have a therapeutic effect on hair loss. Finasteride’s therapeutic efficacy stems from its structural similarity to testosterone. This allows finasteride to competitively bind to 5-alpha reductase, effectively blocking the enzyme’s access to testosterone. Consequently, the production of DHT is significantly diminished, alleviating the detrimental effects of this hormone on hair follicles.
Finasteride has proven to be an effective treatment for androgenetic alopecia, but it can interfere with the normal hormonal processes, potentially causing various side effects, including sexual dysfunction in men.
BACKGROUND KNOWLEDGE
Androgenetic alopecia
and Finasteride
75% of male hair loss patients and 40% of female hair loss patients have androgenetic alopecia.
What is androgenetic alopecia?
Testosterone, a male hormone, is converted into dihydrotestosterone (DHT) by a specific enzyme called 5-alpha reductase. This conversion can lead to the shrinking of hair follicles and a shortened lifespan of hair follicles, resulting in hair loss.
As a solution to this, Merck & Co. in the United States developed ‘finasteride,’ with the idea that reducing DHT production by inhibiting the activity of 5-alpha reductase would have a therapeutic effect on hair loss. This medication has a molecular structure similar to testosterone. It binds to 5-alpha reductase instead of testosterone, preventing the conversion of testosterone into DHT.
Finasteride has shown efficacy in treating hair loss, but it can interfere with the normal hormonal processes, potentially causing various side effects, including sexual dysfunction in men.

BACKGROUND KNOWLEDGE
Androgenetic alopecia and Finasteride
75% of male hair loss patients and 40% of female hair loss patients have androgenetic alopecia.
What is androgenetic alopecia?
The main cause responsible for androgenetic alopecia is dihydrotestosterone (DHT), a more potent form of testosterone. Testosterone, a male hormone, is converted into DHT by a specific enzyme called 5-alpha reductase. DHT binds to hair follicles, causing them to shrink and shorten their growth cycle. This ultimately results in the gradual thinning and eventual loss of hair.
As a solution to this, Merck & Co. developed ‘finasteride,’ with the idea that reducing DHT production by inhibiting the activity of 5-alpha reductase would have a therapeutic effect on hair loss. Finasteride’s therapeutic efficacy stems from its structural similarity to testosterone. This allows finasteride to competitively bind to 5-alpha reductase, effectively blocking the enzyme’s access to testosterone. Consequently, the production of DHT is significantly diminished, alleviating the detrimental effects of this hormone on hair follicles.
Finasteride has proven to be an effective treatment for androgenetic alopecia, but it can interfere with the normal hormonal processes, potentially causing various side effects, including sexual dysfunction in men.

THE BEGINNING OF BLMo-308 RESEARCH
Background of BLMo-308 Research
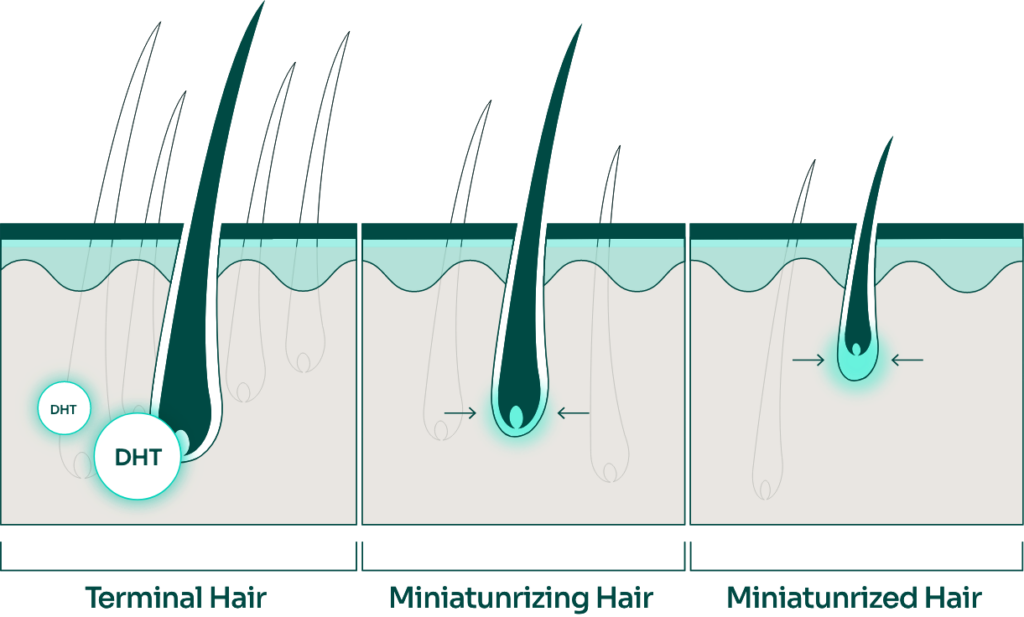
We have advanced beyond the mechanism of finasteride to elucidate that DHT, converted by 5-alpha reductase, increases the inflammatory cytokine IL-6 in dermal papilla cells in the scalp, leading to hair loss.
We thought that by inhibiting the production of IL-6 or by inhibiting the production of 5-alpha reductase itself, hair loss could be prevented. Based on this concept, we developed the ingredient BLMo-308.
THE BEGINNING OF BLMo-308 RESEARCH
Background of BLMo-308 Research
We have advanced beyond the mechanism of finasteride to elucidate that DHT, converted by 5-alpha reductase, increases the inflammatory cytokine IL-6 in dermal papilla cells in the scalp, leading to hair loss.

We thought that by inhibiting the production of IL-6 or by inhibiting the production of 5-alpha reductase itself, hair loss could be prevented. Based on this concept, we developed the ingredient BLMo-308.

THE BEGINNING OF BLMo-308 RESEARCH
Background of BLMo-308 Research
We have advanced beyond the mechanism of finasteride to elucidate that DHT, converted by 5-alpha reductase, increases the inflammatory cytokine IL-6 in dermal papilla cells in the scalp, leading to hair loss.
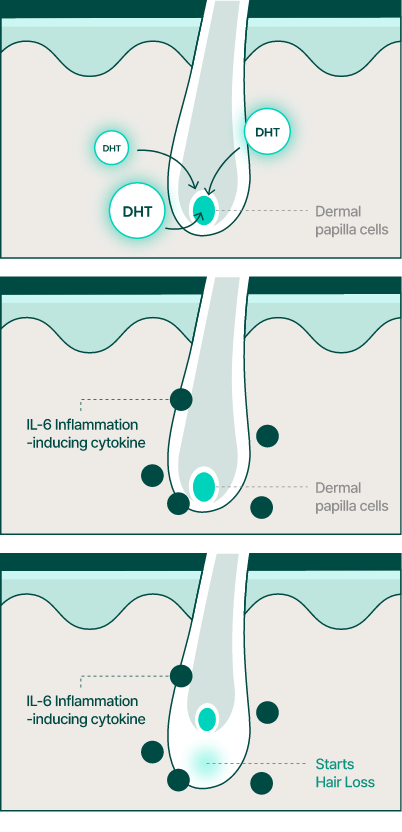
We thought that by inhibiting the production of IL-6 or by inhibiting the production of 5-alpha reductase itself, hair loss could be prevented. Based on this concept, we developed the ingredient BLMo-308.
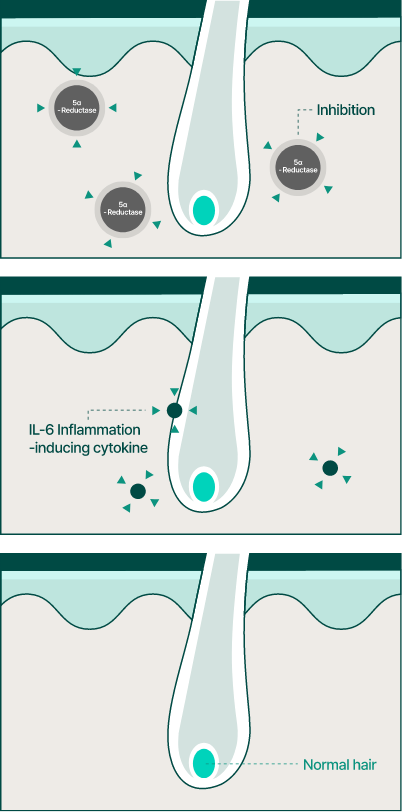
Therefore, BLMo-308 is a versatile hair loss inhibiting ingredient with the potential to be applied to not only to androgenetic alopecia but also to hair loss caused by chronic inflammation.
Therefore, BLMo-308 is a versatile hair loss inhibiting ingredient with the potential to be applied to not only to androgenetic alopecia but also to hair loss caused by chronic inflammation.
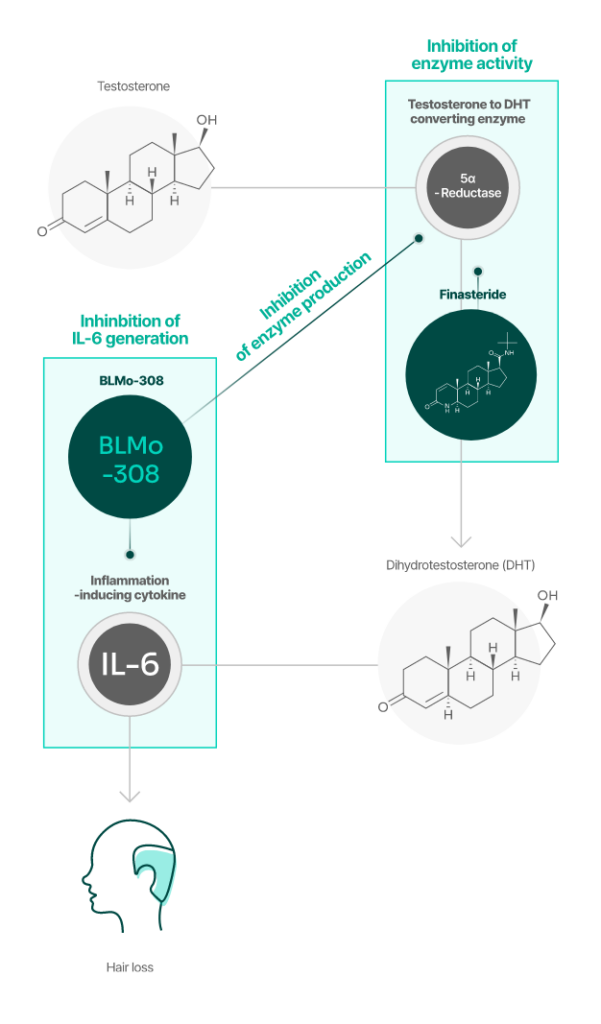
Comparison of the Mechanisms of Action between BLMo-308
and Finasteride
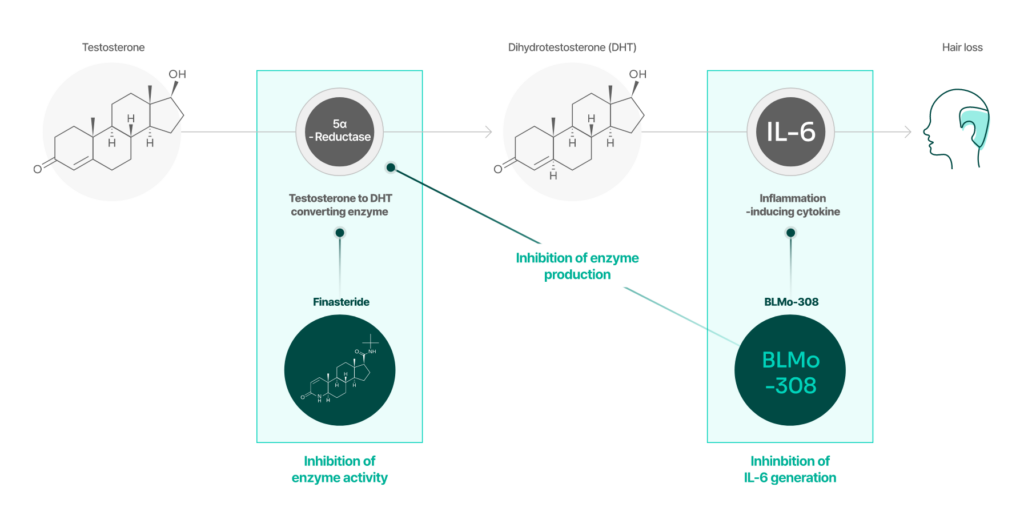
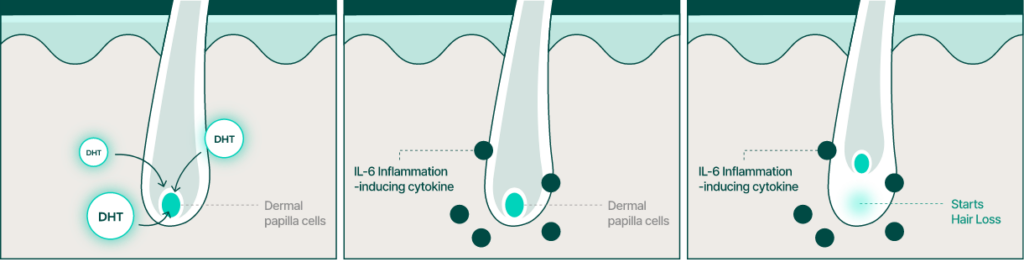
BLMo-308 has a different mechanism of action than finasteride.
BLMo-308
Mechanism: Inhibition of Root Causes
- Inhibition of the Production of 5-Alpha Reductase
- Inhibition of the Production of Inflammatory Cytokine IL-6

Finasteride
Mechanism: Competitive Inhibition
- Competition with Testosterone for binding to the active site of 5-Alpha Reductase
- DHT Formation Inhibition Mechanism
Comparison of the Mechanisms of Action between BLMo-308 and Finasteride

BLMo-308 has a different mechanism of action than finasteride.

BLMo-308
Mechanism: Inhibition of Root Causes
- Inhibition of the Production of 5-Alpha Reductase, Which Converts Testosterone into DHT
- Inhibition of the Production of Inflammatory Cytokine IL-6 That Causes Hair Loss
- Mechanism of Inhibiting the Root Causes

Finasteride
Mechanism: Competitive Inhibition Mechanism
- Finasteride Competes with Testosterone to Bind to 5-alpha Reductase Instead.
- Mechanism of Competitive Inhibition of DHT Production
Comparison of the Mechanisms of Action between BLMo-308 and Finasteride
작용기전이 다릅니다.

BLMo-308
Mechanism: Inhibition of Root Causes
- Inhibition of the Production of 5-Alpha Reductase
- Inhibition of the Production of Inflammatory Cytokine IL-6

Finasteride
Mechanism: Competitive Inhibition
- Competition with Testosterone for binding to the active site of 5-Alpha Reductase
- DHT Formation Inhibition Mechanism
BLMo-308 FROM NATURE
BLMo-308, Natural Ingredient with No Side Effects
BLMo-308 is composed of high-quality plant extracts, not chemical compounds.
It was discovered that the inflammatory signaling molecule IL-6, produced by DHT, is one of the main causes of hair loss. However, finding an ingredient to suppress this from natural substances was a very difficult task, and this process alone took over 10 years.
Finally, in 2015, the effective ingredients were discovered. Then, the process of finding the optimal blending ratios of these ingredients was performed. Through countless trials and errors, we ultimately found the blending ratios that would maximize the therapeutic effect.

BLMo-308 FROM NATURE
BLMo-308, Natural Ingredient with No Side Effects
BLMo-308 is composed of high-quality plant extracts, not chemical compounds.
It was discovered that the inflammatory signaling molecule IL-6, produced by DHT, is one of the main causes of hair loss. However, finding an ingredient to suppress this from natural substances was a very difficult task, and this process alone took over 10 years.
Finally, in 2015, the effective ingredients were discovered. Then, the process of finding the optimal blending ratios of these ingredients was performed. Through countless trials and errors, we ultimately found the blending ratios that would maximize the therapeutic effect.

DEVELOPMENT PROCESS OF BLMo-308
The Development Process of BLMo-308
Although finasteride may have several sex-related side effects and is an absolute contraindication for fertile women, a new medication would be of little commercial significance if it were not more effective than Finasteride in treating hair loss.
Moreover, it should be preceded by the requirement that there are no side effects even with long-term consumption.

① Discovery and Extraction
Based on scientific understanding of natural ingredients and diseases, specific components are extracted and concentrated from plants believed to contain functional active compounds.
② Biochemistry , Cell experiments and Blending
Standardization of ingredients (marker compounds, etc.) is conducted. Biochemical and cellular experiments are employed to identify the best ingredients for hair loss improvement and select the final candidates. Subsequently, efficacy evaluations are performed again with various blending ratios to determine the optimal blending ratio with the most outstanding efficacy.
③ Preclinical Trial
Animal experiments are conduted to evaluate both efficacy and safety, ensuring that the ingredient possesses the intended fuctionality and does not cause any adverse effects on the body even when administered in large quantities. BLMo-308 has also undergone repetitive animal experiments to validate the its efficacy and safety. Based on this, papers have been published in Scopus-indexed journals.
④ Clinical Trial
The final-stage clinical trials must be conducted carefully following Ministry of Food and Drug Safety guidelines. To ensure statistically significant results, a double-blind trial is conducted with a sufficient number of clinical subjects recruited from accredited clinical institution. The results of these clinical trials are reported to the Korea disease control and prevention agency.
From September 2021 to April 2022, a clinical trial was conducted with 101 subjects under the supervision of Professor Lee Ju-hee at the Department of Dermatology, Yonsei University College of Medicine. Subsequently, after the data analysis period, the final clinical trial report was prepared and submitted on December 29, 2022.
DEVELOPMENT PROCESS OF BLMo-308
The Development Process of BLMo-308
Although finasteride may have several sex-related side effects and is an absolute contraindication for fertile women, a new medication would be of little commercial significance if it were not more effective than Finasteride in treating hair loss.
Moreover, it should be preceded by the requirement that there are no side effects even with long-term consumption.

① Discovery, Extraction, and Blending
Based on scientific understanding of natural ingredients and diseases, specific components are extracted and concentrated from plants believed to contain functional active compounds.
② Biochemistry and Cell experiments
Standardization of ingredients (marker compounds, etc.) is conducted. Biochemical and cellular experiments are employed to identify the best ingredients for hair loss improvement and select the final candidates. Subsequently, efficacy evaluations are performed again with various blending ratios to determine the optimal blending ratio with the most outstanding efficacy.
③ Preclinical Tiral
Animal experiments are conduted to evaluate both efficacy and safety, ensuring that the ingredient possesses the intended fuctionality and does not cause any adverse effects on the body even when administered in large quantities. BLMo-308 has also undergone repetitive animal experiments to validate the its efficacy and safety. Based on this, papers have been published in Scopus-indexed journals.
④ Clinical Trial
The final-stage clinical trials must be conducted carefully following Ministry of Food and Drug Safety guidelines. To ensure statistically significant results, a double-blind trial is conducted with a sufficient number of clinical subjects recruited from accredited clinical institution. The results of these clinical trials are reported to the Korea disease control and prevention agency.
From September 2021 to April 2022, a clinical trial was conducted with 101 subjects under the supervision of Professor Lee Ju-hee at the Department of Dermatology, Yonsei University College of Medicine. Subsequently, after the data analysis period, the final clinical trial report was prepared and submitted on December 29, 2022.
THE PRESENT OF BLMo-308
The current status of BLMo-308
BLMo-308 is a product developed using only Food ingredients and is currently sold as a general food.
A clinical trial completed in late 2022 demonstrated that BLMo-308 is effective in improving hair density and diameter. Additionally, no side effects were reported after six months of use. However, we are not satisfied with this and are continuing our research to upgrade it to an even better product.

THE PRESENT OF BLMo-308
The current status of BLMo-308
BLMo-308 is a product developed using only Food ingredients and is currently sold as a general food
A clinical trial completed in late 2022 demonstrated that BLMo-308 is effective in improving hair density and diameter. Additionally, no side effects were reported after six months of use. However, we are not satisfied with this and are continuing our research to upgrade it to an even better product.

THE PRESENT OF BLMo-308
The current status of BLMo-308
BLMo-308 is a product developed using only Food ingredients and is currently sold as a general food.
A clinical trial completed in late 2022 demonstrated that BLMo-308 is effective in improving hair density and diameter. Additionally, no side effects were reported after six months of use. However, we are not satisfied with this and are continuing our research to upgrade it to an even better product.

THE FUTURE OF BLMo-308
Clinical Development Plan for a Novel Drug Candidate
Clinical Development Plan for a Novel Drug Candidate The final clinical results for BLMo-308 were obtained through a double-blind study conducted over six months with participants aged 20 to 59 who were experiencing early-stage hair loss or mild hair damage. Due to the nature of the trial being for a health functional food rather than a new drug, it was not possible to include participants with severe hair loss. Therefore, clinical trials for BLMo-308 had to be conducted with mild hair loss participants selected from volunteers who self-identified or were considered by experts to have not healthy hair.
In the future, as time and resources allow, we plan to conduct clinical trials for a potential new drug candidate. Additionally, even if it is deemed unnecessary to officially register BLMo-308 as a new drug candidate, we intend to further investigate the following clinical results:
[1] We would like to evaluate the hair loss improvement effect after administering BLMo-308 at a 30-50% higher dosage than the current one to patients with moderately severe hair loss for 1 to 5 years.
[2] After a certain period of consumption and improvement in hair loss symptoms, we intend to closely examine whether hair loss will recur when the subjects discontinue the intake and, if so, how long it will take for recurrence.
[3] Based on the current clinical results, it appears that 34 out of 44 trial subjects have shown improvement or maintenance of their condition. However, it remains a question as to what might be the cause of the approximately 25% of subjects who did not show any improvement. It is possible that the intake dosage may not have been sufficient to alleviate their symptoms, or they may have had different underlying causes for their hair loss symptoms that were unrelated to the mechanisms targeted by BLMo-308. We would like to confirm these factors more accurately.
THE FUTURE OF BLMo-308
Clinical Development Plan for a Novel Drug Candidate
Clinical Development Plan for a Novel Drug Candidate The final clinical results for BLMo-308 were obtained through a double-blind study conducted over six months with participants aged 20 to 59 who were experiencing early-stage hair loss or mild hair damage. Due to the nature of the trial being for a health functional food rather than a new drug, it was not possible to include participants with severe hair loss. Therefore, clinical trials for BLMo-308 had to be conducted with mild hair loss participants selected from volunteers who self-identified or were considered by experts to have not healthy hair.
In the future, as time and resources allow, we plan to conduct clinical trials for a potential new drug candidate. Additionally, even if it is deemed unnecessary to officially register BLMo-308 as a new drug candidate, we intend to further investigate the following clinical results:
THE FUTURE OF BLMo-308
Clinical Development Plan for a Novel Drug Candidate
Clinical Development Plan for a Novel Drug Candidate The final clinical results for BLMo-308 were obtained through a double-blind study conducted over six months with participants aged 20 to 59 who were experiencing early-stage hair loss or mild hair damage. Due to the nature of the trial being for a health functional food rather than a new drug, it was not possible to include participants with severe hair loss. Therefore, clinical trials for BLMo-308 had to be conducted with mild hair loss participants selected from volunteers who self-identified or were considered by experts to have not healthy hair.
In the future, as time and resources allow, we plan to conduct clinical trials for a potential new drug candidate. Additionally, even if it is deemed unnecessary to officially register BLMo-308 as a new drug candidate, we intend to further investigate the following clinical results: