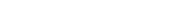Clinical Trial Report
Efficacy was found in 75% of participants who consumed BLMo-308.
The clinical trial of BLMo-308 was conducted from September 2021 to April 2022 at the Global
Medical Research Center, under the supervision of Professor Joo-hee Lee at the Department of
Dermatology, Yonsei University College of Medicine. A total of 101 participants were evaluated for
efficacy and safety simultaneously.




Hair Density
[Interpretation of Trial Results]
1. Statistically significant differences in hair density was observed from the 8th week (2 months) of BLMo-308 consumption.
2. The trend of widening differences between the test group and the control group continued until week 24 (6 months). Since it was a 6-month clinical trial, measurements were not taken beyond this point, but there is a possibility that the difference between the two groups would continue to expand.
3. The between groups difference in hair density at 6 months showed a significant p-value of 0.0006.
4. In people with hair loss in progress, it is estimated that the difference in hair count between not taking BLMo-308 and taking BLMo-308 over a six-month period could be approximately 680 hairs (assuming a total scalp area of 200 cm2).
5. Among the test group, a hair density improvement effect was observed in approximately 75% of the participants.

Hair Diameter (Thickness)
[Interpretation of Trial Results]
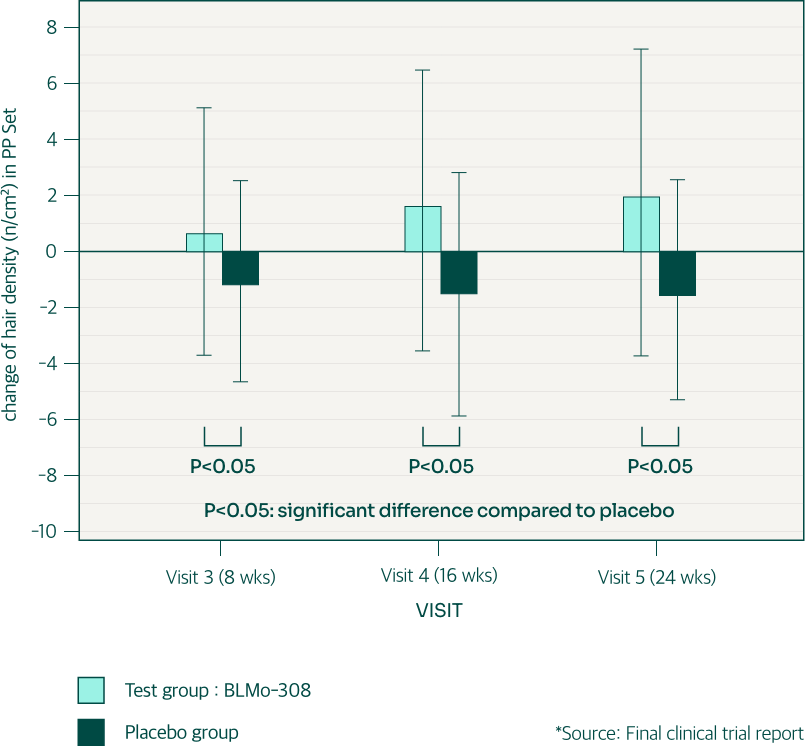
1. Statistically significant differences between the test and control groups were observed starting at the 16th week (4 months) of BLMo-308 intake.
2. This trend of an increasing difference continued up to 24 weeks (6 months). Specifically, hair diameter in the test group increased by about 5% after 6 months of intake compared to baseline, while the control group saw a decrease of about 1%.
3 . The difference in hair diameter shows a significant p-value of 0.0001 at 6 months.
4. In the test group, approximately 75% of participants showed an increase in hair diameter.
Most of those who saw improvement in hair density also exhibited an increase in hair diameter. Therefore, the consistency of hair health improvement effect is very high.

Gloss of Newly Grown Hair
[Interpretation of Trial Results]
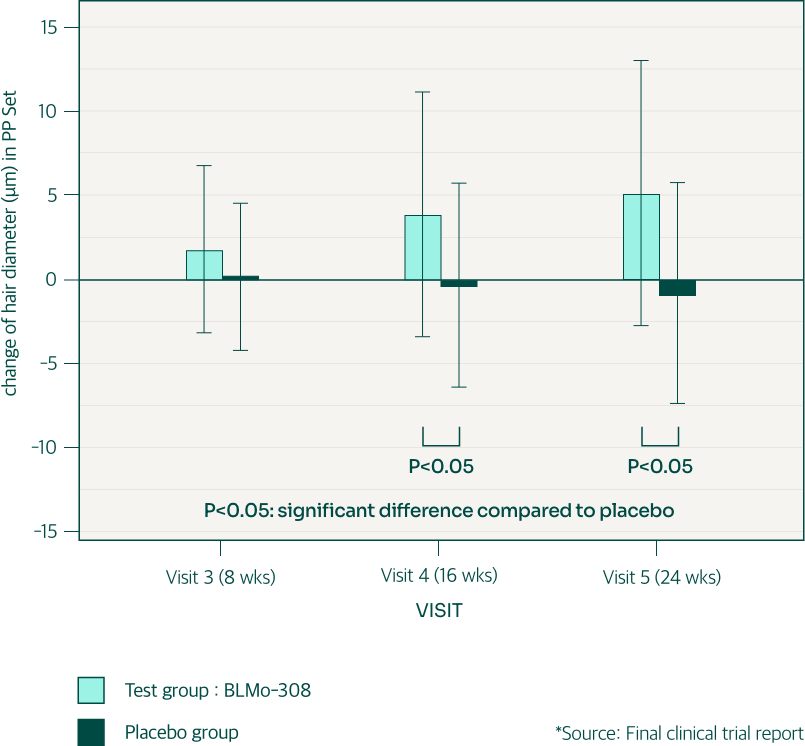
To minimize the influence of external factors (shampoo, conditioner, etc.), this clinical trial evaluated the gloss of newly grown hair. After 8 weeks of consumption, the BLMo-308 group showed a significant improvement in hair gloss compared to the control group. However, this difference disappeared after 6 months.
This initial increase in hair gloss suggests BLMo-308 may directly benefit newly grown hair, which is less affected by external factors like hair care products. However, over time, external factors likely influenced the control group’s hair gloss, reducing the initial difference between the groups.

BLMo-308 Article Published
Clinical results of BLMo-308 have been published in a SCI-grade journal.
BLMo-308 Article Published
Clinical results of BLMo-308 have been published in a SCI-grade journal.
BLMo-308 Article Published
Clinical results of BLMo-308 have been published in a SCI-grade journal.

Efficacy and safety of persimmon leaf formulated
with green tea and sophora fruit extracts (BLH308) on hair growth:
A randomized, double-blind, placebo-controlled clinical trial
Seoyoon Ham, Young In Lee, In Ah Kim, Jangmi Suk, Inhee Jung,
Jong-Moon Jeong, Ju Hee Lee