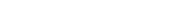More information
More information
Learn more about the clinical trial results of BLMo-308.
Learn more about the clinical trial results of BLMo-308.
The clinical trial of BLMo-308 was conducted from September 2021 to April 2022 at the Global Medical Research Center, under the supervision of Professor Lee Joo-hee at the Department of Dermatology, Yonsei University College of Medicine. A total of 101 participants were evaluated for efficacy and safety simultaneously.
Each Participant participated in a 24-week randomized, double-blind, placebo-controlled clinical trial. In this type of trial, neither the participants nor the researchers knew whether the substance being administered was the test food or a placebo.

"Conducted with rigorous security under a double-blind approach, ensuring that even the investigators were unaware."
"Conducted with rigorous security under a double-blind approach, ensuring that even the investigators were unaware."
Out of the participants who met the selection criteria set by the clinical institution, including various physical conditions and medication histories, a total of 88 individuals were smoothly adhered to the designated protocol from the beginning to the end of the trial.
Among them, the test group, which actually consumed the test substance (BLMo-308), consisted of 44 individuals (27 females and 17 males), while the controlled group, which received a placebo substance, also included 44 individuals.

A total of 88 subjects completed the entire process, with 44 individuals in both the test and control groups.
A total of 88 subjects completed the entire process, with 44 individuals in both the test and control groups.
Throughout the 6-month program, all clinical participants visited the institution every 8 weeks for a total of 4 visits. During these visits, various measurements were taken to assess scalp condition, hair status, and other clinicopathological conditions.
The participants in this clinical trial were individuals aged 19 to 60, who had mild to moderate hair damage. They were identified based on gloss score of 1 or more and 3 or less according to the visual evaluation classification method, and the total score for hair damage of less than 18 points according to the assessment of exposure to risk factors.
A total of 88 participants (PP group), who met these rigorous criteria and consistently followed the designated protocol from the beginning to the end of the trial, were selected. For these participants, various measurements including changes in hair density, hair diameter, hair gloss, hair elasticity, scalp moisture changes, and participant satisfaction were conducted. The final measurement results and scores were analyzed using various statistical methods recommended by the Ministry of Food and Drug Safety to determine p-values and identify significant differences between the test and control groups.
The P-value is the key indicator that clearly reflects the difference between the test and control groups. Generally, when the P-value is below 0.05, it can be concluded that the groups are statistically significantly different. In fact, the lower the P-value is below 0.05, the more pronounced the difference between the test and control groups, indicating that the effect of the test group is significantly superior.
The clinical results of BLMo-308 regarding hair density measurements showed that, in comparison between the test and control groups, after just 2 months of BLMo-308 consumption, the P-value had already dropped significantly below 0.05. This trend continued even after 4 months and 6 months of consumption, with the P-value remaining significantly lower than 0.05.

P-value by Wilcoxon method = 0.0015
P-value by GLM (ANCOVA) method = 0.0006
P-value by Wilcoxon method = 0.0015
P-value by GLM (ANCOVA) method = 0.0006
*Note: The Wilcoxon method, GLM (ANCOVA) method, T-test method, etc., are methods of calculating P-values for measured results. There are some differences depending on the P-value calculation method, but all results are significant at less than 0.05.
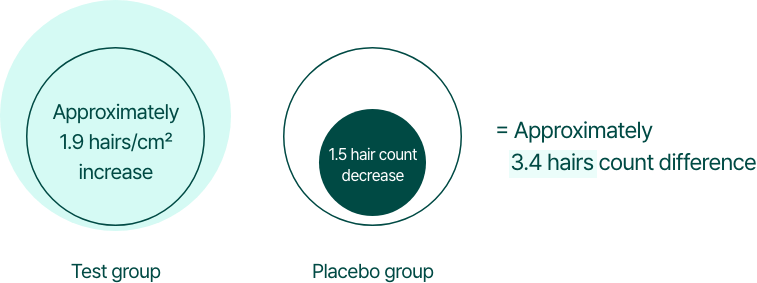
Furthermore, out of the 44 participants in the test group, 34 participants experienced an increase or maintenance in hair density compared to before the start of the trial, indicating that 75% of the test group had improved hair density.
By the 6th month of consumption, the test group exhibited an increase of approximately 1.9 hairs/cm², while the control group showed a decrease of 1.5 hairs/cm², resulting in an approximate 3.4 hairs/cm² difference between the two groups.
If we assume that the total scalp area of a person is approximately 200 cm², then depending on whether they consume BLMo-308, there would be a difference of around 680 hairs.
This suggests that it had a significantly positive effect, particularly for individuals with mild hair loss symptoms.
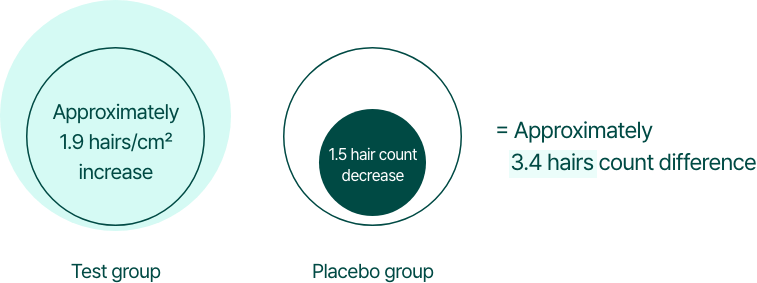

P-value by 2 Sample T-test method = 0.0001
P-value by GLM (ANCOVA) method = 0.0001
P-value by 2 Sample T-test method = 0.0001
P-value by GLM (ANCOVA) method = 0.0001
Actually, in the test group, when measuring the average hair diameter at the 6th month of consumption, it increased by approximately 5% compared to before the start of the trial. In contrast, the control group showed a decrease of about 1% when compared to before the start of the trial. As a result, the difference in hair diameter between the test and control groups amounted to a total of approximately 6%.
In the results of hair diameter measurements, the P-value dropped below 0.05, indicating statistical significance, starting from the 4th month of BLMo308 consumption. By the 6th month of consumption, the P-value showed 0.0001, demonstrating a highly significant difference.
Furthermore, it was observed that approximately 75% of the participants had increased hair diameter. It indicates that participants whose hair diameter increased also had increased hair density.


Overall Clinical Trial Evaluation
The recent clinical results of BLMo-308 have been published in Skin Research & Technology. The fact that such clinical results were achieved by blending extracts from specific plants commonly used as food is remarkably surprising, and we believe that this product has the potential to be a very encouraging solution for individuals dealing with hair loss.
Finasteride, the active ingredient in Propecia, has been the participant of extensive clinical research since the late 1990s, involving hundreds, and even thousands, of participants in large hospitals worldwide and its efficacy has been published in clinical journals. They typically conduct clinical trials lasting from 1 year to 5 years, during which they report the changes in hair density, subjective evaluation of hair density improvement from clinical trial participants, and accompanying side effects.
However, while consuming products made from health functional food ingredients like BLMo-308…
1. With the supervision of a dermatology professor from the medical school, a scientific study was designed involving close to 100 clinical participants,
2. To ensure the safety of clinical participants, rigorous procedures were followed, including approval from an Institutional Review Board (IRB),
3. The results of each measurement were statistically analyzed to determine whether there is a significant difference,
4. It is quite exceptional that during the clinical trial, it was demonstrated that adverse events experienced by some participants were entirely unrelated to the consumption of BLMo-308, further affirming its exceptional safety for human consumption.
BLMo-308 is an ingredient suitable for individuals with early-stage hair loss,
including androgeneticalopecia.
Statistically, androgenetic alopecia accounts for approximately 75% of male pattern baldness and 40% of female pattern baldness cases.
Therefore, it can be assessed that there was a significant effect on participants with androgenetic
alopecia and those with mild hair loss of unknown cause who participated in the trial.