What are the side effects of finasteride as reported in clinical studies and other review papers, and what are their severity and average incidence rates?
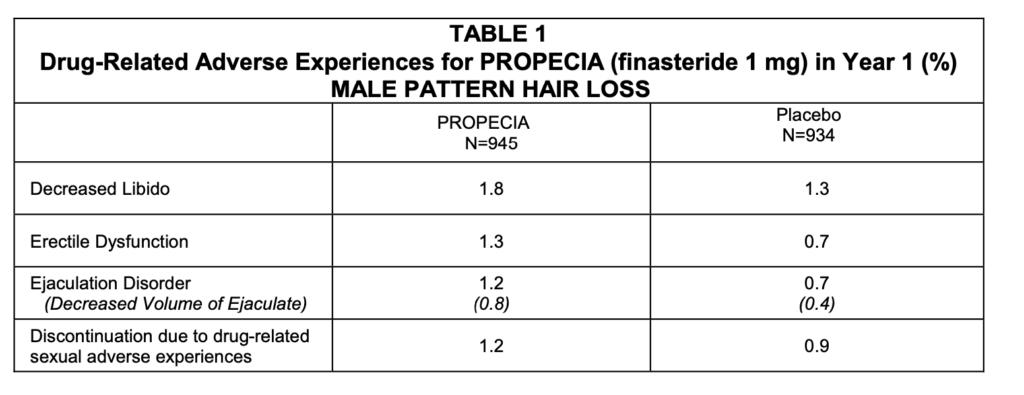
The results shown in the following table are side effects of finasteride directly announced by Merck, based in New Jersey, USA, in December 2010. Merck’s data may not capture the full range of side effects, so it’s important to consider data from other sources as well.
Since sexual issues also occurred in the placebo group, the adjusted rates are reported as follows: decreased libido at 0.5%, erectile dysfunction at 0.6%, decreased ejaculate volume at 0.5%, and discontinuation during intercourse at 0.3%. Recently, the U.S. Food and Drug Administration (FDA) sent a notice to all countries where finasteride is sold, mandating that they include a warning about the risk of suicidal ideation due to depression in the drug’s side effects information. This indicates that finasteride not only has a significant incidence of sexual dysfunction-related side effects but also has clear evidence of causing complex psychological side effects, including depression and suicidal ideation, by affecting brain function.