How does Olumiant (baricitinib) treat alopecia areata?
Recently, the U.S. Food and Drug Administration (FDA) approved Olumiant (baricitinib), originally a rheumatoid arthritis medication, as a treatment for alopecia areata. Let's explore the mechanism of action, potential side effects, and treatment efficacy of Olumiant.

Olumiant (baricitinib) was initially developed as a treatment for rheumatoid arthritis. Its efficacy lies in its ability to modulate the JAK-STAT signaling pathway, which plays a crucial role in regulating the body’s inflammatory response.
JAK (Janus kinase) proteins act as intermediaries, transmitting signals from cytokines (small signaling molecules) to STAT (signal transducer and activator of transcription) proteins. Upon activation by cytokines, JAKs phosphorylate STAT proteins, enabling them to enter the cell nucleus and trigger the expression of various genes involved in blood cell formation, inflammation, and immune responses. Olumiant, as a JAK inhibitor, selectively blocks the phosphorylation of JAK proteins, thereby disrupting this signaling pathway. Without activation, STAT proteins cannot enter the cell nucleus, and the transcription of inflammatory genes is suppressed. Consequently, Olumiant exerts its therapeutic effect in rheumatoid arthritis by dampening the inflammatory response.
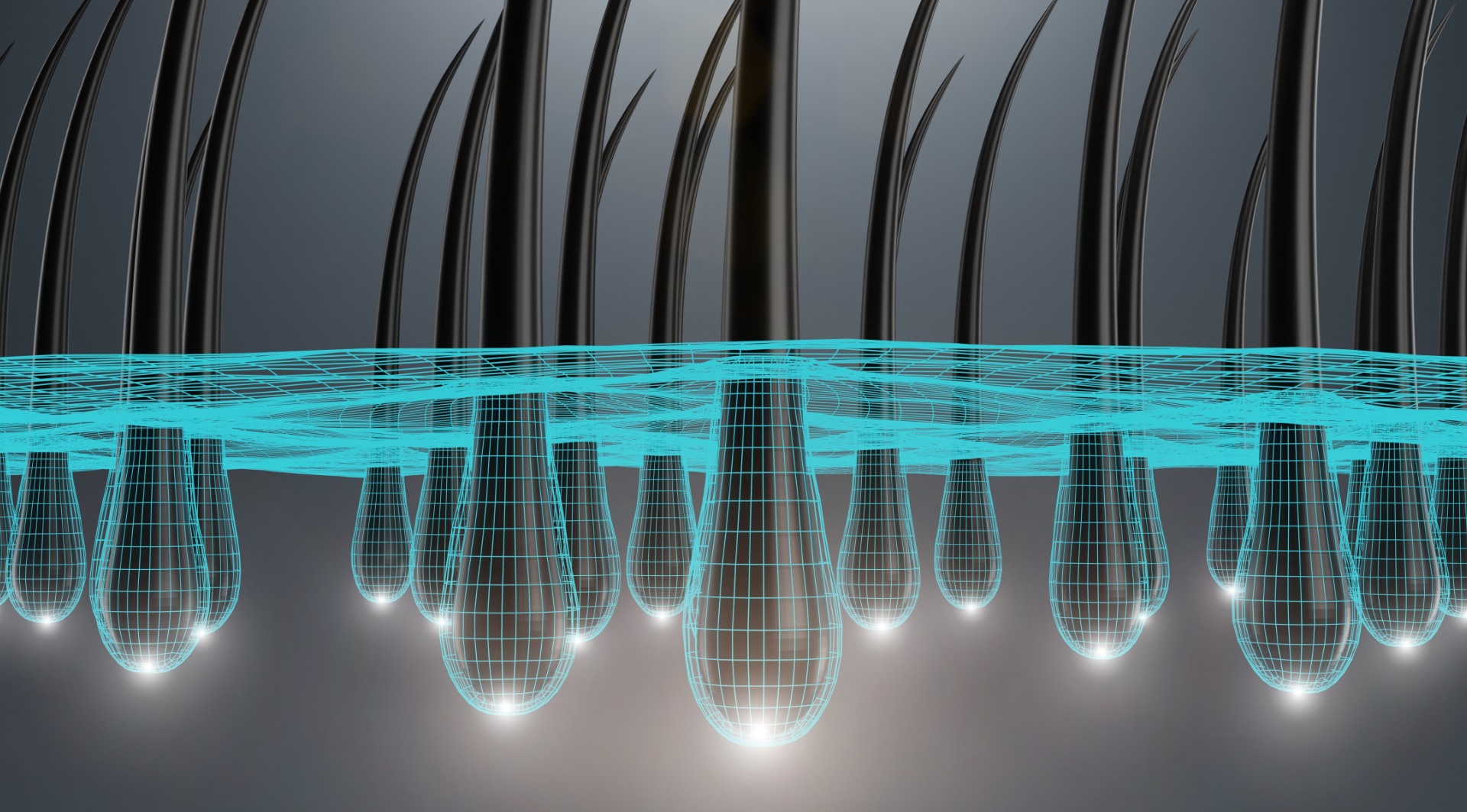
The efficacy of Olumiant extends beyond rheumatoid arthritis, demonstrating promising results in the treatment of ulcerative colitis and atopic dermatitis. More recently, it has gained recognition for its potential in treating alopecia areata, an autoimmune condition characterized by hair loss in circular patches caused by the immune system mistakenly attacking hair follicles, leading to inflammation and disrupted hair growth.
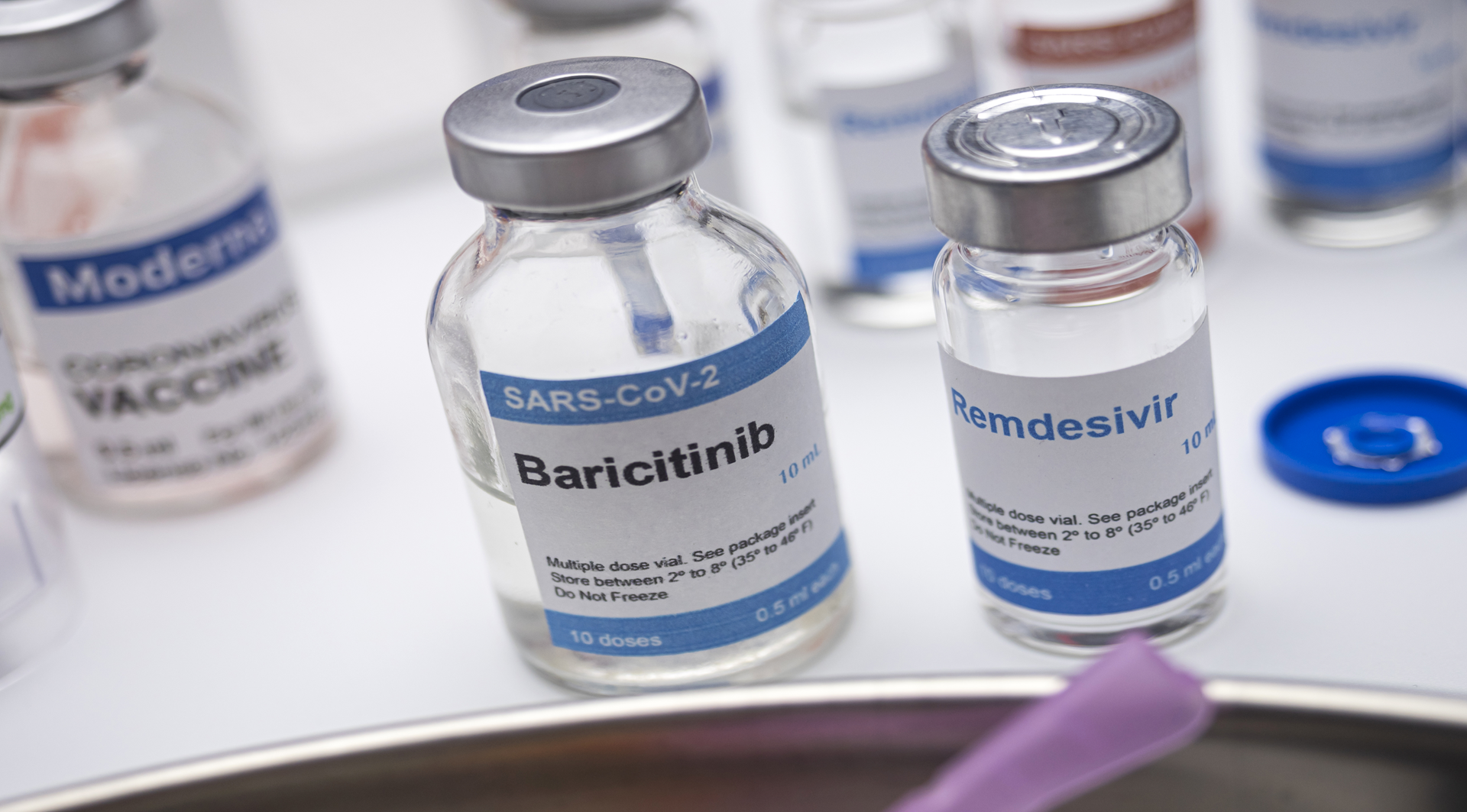
The development of Olumiant as a treatment for alopecia areata originated from research into COVID-19 therapies. During clinical trials for COVID-19 treatment, some patients experienced alopecia areata as a side effect associated with excessive inflammation due to severe COVID-19. Interestingly, Olumiant not only aided in managing COVID-19 symptoms but also showed improvement in this hair loss condition.
Intrigued by these observations, researchers delved into exploring Olumiant’s potential for alopecia areata treatment. Olumiant’s mechanism of action, targeting JAK inhibition, reduces the inflammatory response that attacks hair follicles, potentially restoring hair follicle function and enabling hair regrowth. Clinical trials indeed supported this mechanism, as patients treated with Olumiant experienced significant hair regrowth compared to the placebo group.
Supported by these clinical outcomes, Olumiant received FDA approval in 2022 as the first oral systemic treatment for adult alopecia areata.